OVERVIEW
Complete eCOA solutions for clinical trials of all sizes
Clario’s enhanced eCOA study build, driven by science, delivers rapid setup and deployment to help meet critical deadlines through:
Simplified design specification review
Greater configuration
Faster translations
Pre-built, validated standardized assessment libraries
Clario’s vast experience in eCOA services includes:
- 25 years of DCT and hybrid trials
- A truly global scale with studies in over 120 countries and over 100 languages
- Flexible technology options, including bring-your-own-device (BYOD), web or provisioned device
- Configurable platform adapts to your trial needs
FREE ESTIMATION TOOL
Understand the starting cost of using paper PROs
With paper, you could be paying more for less.
See if paper is draining your budget.
Before your next trial, use our paper-cost calculator to estimate the potential starting expense of your patient-reported outcomes (PROs).
SOLUTIONS
Clario’s eCOA Platform
Our world-class scientific expertise drives solutions which are built around your patient, powered by innovative technology, flexible device modalities, and supported by over 25 years of experience.
ACCESSIBILITY FEATURE
Zoom In/Zoom Out
Enhance inclusivity while maintaining data integrity
- Increase accessibility:
Include individuals with visual impairments who have a difficult time completing assessments. - Reduce bias:
Minimize the need for caregiver assistance. - Reduce burden:
Eliminate the need to remember reading aids.
- Collect high-quality data:
Extensively tested, science-led design that demonstrates equivalency and ensures all answer options are visible to participants. - Support improved compliance:
Provide a better participant user experience.
Connected devices with eCOA
Make taking part in clinical studies straightforward
Clario’s devices are pre-validated for use in clinical trials and integrated with the eCOA device to capture date ECG, spirometry, blood glucose levels, activity levels, movement endpoints and many more.
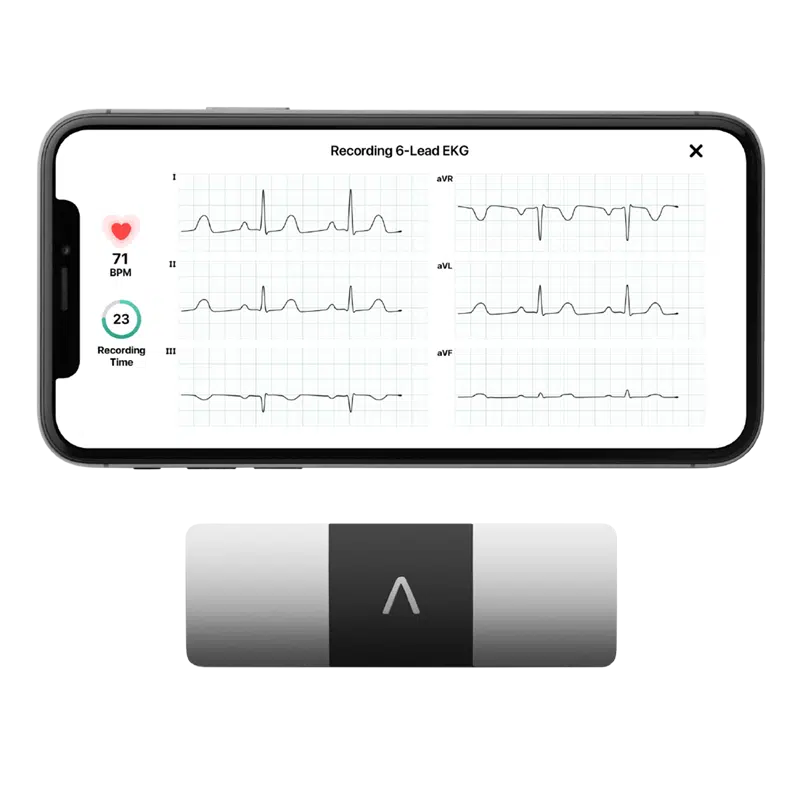
Alivecor KardiaMobile6L

iSpiro®

Glucometer

Actigraph Watch

Opal® Precision Motion Monitoring
Flexible device modalities
Familiar devices, streamlined experience
Empower patients to participate in trials from anywhere using their smartphones or tablets, simplifying data collection and leading to faster enrollment. Clario’s secure platform ensures the highest quality data, giving you the insights you need for successful drug differentiation, development and regulatory approval.
Provisioned devices
Tablets
BYOD
Home computer
TEAM
Scientific guidance and therapeutic area solutions
Engage with our eCOA scientific and therapeutic area experts early on in your clinical development program to ensure the right eCOA endpoints are being measured, using the most appropriate assessments and the right technology. Our team has developed tailored solutions across multiple therapeutic areas and indications built upon 25 years of industry experience.
Our therapeutic area expertise across 560 indications

Endocrinology
Immunology
Vaccines
Women’s Health
Talk to a specialist
Our team of experts is available to address questions you may have about our integrated eCOA solutions. Submit your contact information and we’ll be in touch shortly.









